Selecting Hygienic Roll Crushers for Food Grade Calcium Carbonate Production
The production of food grade calcium carbonate demands equipment that meets exceptionally high standards of hygiene and material safety. Unlike industrial crushing applications, food grade processing requires careful consideration of microbial control, material compatibility, and cleanability throughout the equipment design. This comprehensive guide examines the critical factors in selecting roll crushers specifically for food grade calcium carbonate applications, addressing everything from surface finish requirements to validation documentation. Understanding these requirements ensures compliance with global food safety standards while maintaining optimal processing efficiency and product quality.
Understanding Special Hygiene Requirements for Food Grade Calcium Carbonate Production
Food grade calcium carbonate serves as both a nutrient supplement and processing aid in numerous food products, making purity and safety paramount concerns. The crushing equipment used in its production must prevent contamination while effectively reducing particle size to specification. Microbial control represents a particular challenge in crushing operations where moisture and organic matter could potentially create breeding grounds for bacteria and mold. The equipment design must facilitate thorough cleaning and sanitation while resisting corrosion from cleaning chemicals and process materials.
Modern food safety standards require a preventive approach to contamination control rather than relying solely on cleaning procedures. This philosophy extends to all aspects of equipment design, including material selection, surface finishes, and component integration. The crushing environment presents unique challenges due to the generation of fine powders that can accumulate in crevices and hard-to-reach areas. Proper equipment selection must address these concerns through designs that minimize particle entrapment while allowing complete access for cleaning and inspection. Regulatory compliance forms just one aspect of the selection criteria, with practical cleanability and maintenance requirements playing equally important roles in long-term operational success.
Regulatory Standards and Equipment Compliance for Food Grade Applications
Global regulatory frameworks establish specific requirements for equipment used in food processing applications. These standards address material safety, design principles, and cleaning protocols to ensure final product purity. The selection process must consider regional variations in regulatory requirements while aiming for the highest common denominator to facilitate international market access. Documentation requirements extend beyond initial certification to include ongoing validation and record-keeping throughout the equipment lifecycle.
Microbial Control Through Strategic Equipment Design
Preventing microbial contamination requires designs that eliminate habitats where microorganisms could establish and multiply. Surface imperfections, crevices, and stagnant areas can harbor bacteria despite regular cleaning procedures. Modern hygienic equipment addresses these concerns through smooth, continuous surfaces and proper drainage capabilities. The relationship between equipment design and microbial risk management forms a critical consideration in selection criteria for food grade processing equipment.
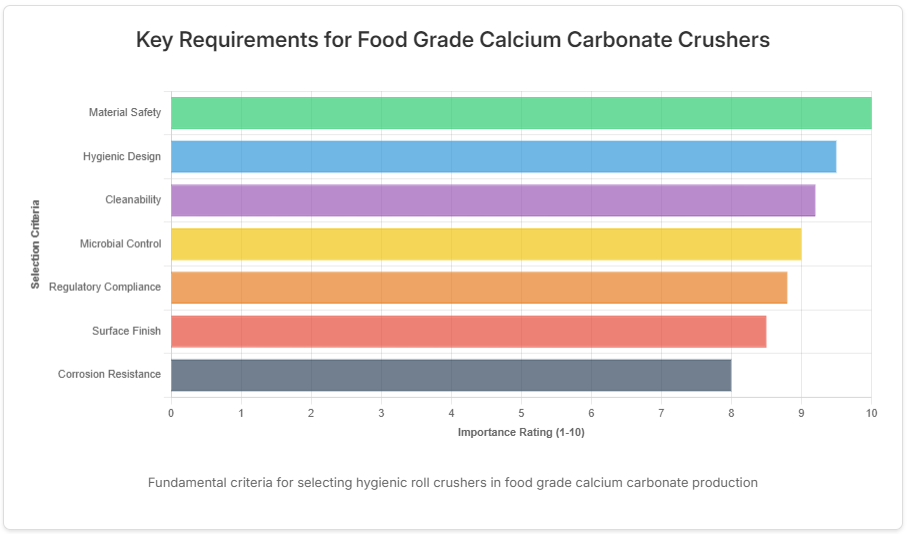
Core Design Standards for Hygienic Roll Crushers
Hygienic Equipment evaluation criteria for food grade calcium carbonate production
Comparison of key performance metrics between standard industrial roll crushers and hygienic design crushers for food grade applications
Hygienic roll crushers incorporate specific design features that distinguish them from standard industrial crushing equipment. These machines prioritize cleanability and contamination prevention through carefully engineered components and configurations. Surface characteristics play a particularly important role, with specific finish requirements that prevent bacterial adhesion while resisting corrosion from both processing materials and cleaning agents. The fundamental design philosophy centers on creating equipment that can be thoroughly cleaned and inspected with minimal disassembly requirements.
Modern hygienic crusher designs incorporate rounded corners and smooth transitions between components to eliminate areas where material could accumulate. The internal geometry ensures complete drainage while preventing the formation of pockets or dead spaces. These design features work together to create equipment that supports rather than complicates cleaning protocols. The selection process must carefully evaluate how each design element contributes to the overall hygiene strategy, considering both initial compliance and long-term maintenance requirements.
Surface Finish Specifications and Their Practical Implications
Surface roughness measurements provide quantifiable data for evaluating equipment cleanability. The Ra value, representing arithmetic average roughness, serves as a key specification for food contact surfaces. Lower Ra values correlate with improved cleanability and reduced bacterial adhesion, making this specification particularly important for equipment used in food grade applications. Specific finishing processes achieve the required surface characteristics while maintaining material integrity and corrosion resistance.
Dead Space Elimination Through Advanced Engineering
Equipment designs must minimize or eliminate areas where material can accumulate and evade cleaning procedures. This requires careful attention to component interfaces, fastening methods, and internal geometries. Modern manufacturing techniques enable seamless transitions between components, while specialized fastening systems provide secure connections without creating contamination risks. The engineering approach prioritizes simplicity and accessibility without compromising structural integrity or operational efficiency.
Material Selection and Surface Treatment Technologies
Material compatibility forms the foundation of hygienic equipment design for food grade applications. Austenitic stainless steels, particularly specific grades with enhanced corrosion resistance, serve as the primary material for food contact surfaces. These materials must withstand both the processing environment and aggressive cleaning protocols while maintaining their structural and surface characteristics. The selection process involves evaluating numerous factors beyond basic corrosion resistance, including mechanical properties, fabrication characteristics, and long-term performance.
Surface treatments enhance material performance while improving cleanability and durability. Passivation processes optimize the corrosion resistance of stainless steels by promoting the formation of a protective oxide layer. Electropolishing simultaneously improves surface smoothness and corrosion resistance through controlled electrochemical removal of surface material. These treatments must be properly specified and validated to ensure consistent results that meet the stringent requirements of food grade processing applications.
Stainless Steel Grade Selection for Optimal Performance
The specific grade of stainless steel significantly impacts equipment performance in food processing environments. Chromium and nickel content influence corrosion resistance, while carbon content affects weldability and sensitization resistance. Molybdenum additions enhance resistance to pitting corrosion in chloride-containing environments. These compositional factors must be balanced against mechanical requirements and fabrication considerations to select the optimal material for each application.
Non-Metallic Materials in Food Contact Applications
Polymer materials and specialized coatings play important roles in hygienic equipment design where their specific properties offer advantages over metals. These materials must demonstrate compliance with food contact regulations while providing the necessary mechanical and chemical resistance. Certification requirements ensure that these materials do not transfer harmful substances to food products under normal use conditions. The selection process involves careful evaluation of temperature resistance, mechanical properties, and cleanability characteristics.
Integrated Cleaning and Sanitation System Design
Modern hygienic equipment designs incorporate cleaning considerations as fundamental elements rather than afterthoughts. Clean-in-place systems allow thorough cleaning without disassembly, significantly reducing downtime while improving cleaning consistency. These systems must provide complete coverage of all product contact surfaces while operating within practical parameters for flow rates, pressures, and chemical concentrations. The integration of these systems requires careful planning during equipment design to ensure effectiveness without compromising structural integrity or operational efficiency.
Sanitation procedures extend beyond basic cleaning to include disinfection and drying steps that prevent microbial growth between production cycles. Equipment design must support these processes through proper drainage, accessible sampling points, and materials compatible with cleaning chemicals. The relationship between equipment design and cleaning efficiency represents a critical consideration in selection criteria, with significant implications for operational costs and product safety.
Clean-in-Place System Optimization for Crushing Equipment
Effective CIP systems require careful nozzle selection and placement to ensure complete coverage of all internal surfaces. The physical properties of cleaning solutions, including temperature, viscosity, and chemical activity, influence cleaning effectiveness and must be considered in system design. Flow dynamics within the equipment affect how cleaning solutions contact surfaces and remove soil materials. These factors must be balanced to create efficient cleaning protocols that minimize resource consumption while ensuring thorough contamination removal.
Accessibility Features for Manual Cleaning and Inspection
Despite advances in automated cleaning systems, certain equipment aspects still require manual attention for thorough cleaning and inspection. Quick-disconnect fasteners and hinged components facilitate access to internal areas without requiring special tools or extensive disassembly. These features must provide secure operation during processing while enabling easy access for maintenance activities. The design should minimize the number of separate components and fasteners to reduce the risk of contamination from lost or misplaced parts.
Certification and Compliance Documentation Management
Regulatory compliance requires comprehensive documentation covering materials, design, fabrication, and testing. Certification bodies establish specific requirements for equipment used in food processing, with variations between different regulatory jurisdictions. Understanding these requirements helps manufacturers design equipment that meets global standards while facilitating market access. Documentation management extends throughout the equipment lifecycle, from initial design through ongoing maintenance and modification.
Validation protocols demonstrate that equipment meets its intended design specifications and performs reliably under actual operating conditions. These protocols include installation qualification, operational qualification, and performance qualification stages that systematically verify equipment capabilities. Proper documentation provides evidence of compliance while supporting troubleshooting and continuous improvement efforts. The selection process should consider not only initial certification but also the ongoing documentation requirements for maintaining compliance.
International Standards and Certification Systems
Multiple organizations establish standards for hygienic equipment design and fabrication, each with slightly different emphases and requirements. Understanding the similarities and differences between these standards helps manufacturers design equipment that meets broad market needs. Certification processes involve independent verification that equipment meets applicable standards, providing confidence to end users and regulatory authorities. The selection process should consider which certifications are most relevant to the target markets and applications.
Documentation Requirements for Equipment Validation
Comprehensive documentation provides the foundation for equipment validation and ongoing compliance. Material certifications verify that all food contact materials meet relevant safety standards. Design documentation demonstrates compliance with hygienic design principles and functional requirements. Fabrication records provide evidence that equipment was constructed properly using approved materials and processes. Together, these documents form a complete package supporting equipment qualification and regulatory compliance.
Performance Validation and Continuous Improvement Systems
Equipment validation begins during factory acceptance testing and continues through installation and initial operation. Performance testing under controlled conditions verifies that equipment meets its design specifications for both processing efficiency and hygienic operation. These tests must simulate actual operating conditions while allowing careful monitoring and data collection. The validation process provides baseline data for comparison during future operation and helps identify potential issues before they affect product quality.
Continuous improvement systems use operational data to identify opportunities for enhancing equipment performance and reliability. Trend analysis helps predict maintenance needs before they result in downtime or quality issues. Performance monitoring provides data for optimizing operating parameters and cleaning protocols. These systems transform validation from a one-time event into an ongoing process that supports long-term operational excellence.
Factory Acceptance Testing for Hygienic Equipment
Factory testing provides the first opportunity to verify equipment performance under controlled conditions. These tests evaluate mechanical operation, processing capability, and cleanability using standardized protocols. Testing under load conditions verifies that equipment can handle the intended materials while maintaining proper operation. Cleanability testing demonstrates that all product contact surfaces can be effectively cleaned using the specified procedures. The results establish baseline performance data for comparison during subsequent validation stages.
Operational Qualification in Production Environments
Site-based testing verifies that equipment performs properly within the actual production environment. These tests confirm proper installation and integration with supporting systems and utilities. Operational testing under production conditions validates performance with actual materials and processing parameters. Cleaning validation demonstrates that sanitation procedures effectively maintain equipment in a hygienic condition between production cycles. Successful completion of these tests provides confidence that the equipment will perform reliably during routine operation.